SINGAPORE, Nov 14, 2013 - (ACN Newswire) - Scientists at A*STAR's Singapore Immunology Network (SIgN) have discovered the exact mode of action by plerixafor, a drug commonly prescribed to stimulate immune responses in patients suffering from neutropenia, which causes them to become prone to oral, skin, genital infections and in worst cases, a fatal whole-body infection(1). A better understanding of the drug's mechanism can improve its usage to more effectively reduce risk of infections in these patients.
 | | A*STAR Scientists Bring to Light Mechanism of Drug for Infections |
Scientists at SIgN employed cutting-edge imaging techniques to analyze the effects of plerixafor on the white blood cell activity in the study which was published in the Journal of Experimental Medicine (JEM).
Neutrophil Mobilization via Plerixafor
Neutropenia is a condition characterized by the lack of a type of white blood cells, also known as neutrophils(2), in one's blood circulation. Plerixafor increases the concentration of these white blood cells in the blood by inhibiting a protein called CXCR4. This inhibition prevents neutrophils in the blood stream from returning to the bone marrow, which is the primary compartment where the white blood cells are stored and released. It is therefore commonly accepted that the efficacy of the drug arises only from the release of these white blood cells from the bone marrow.
However, scientists at SIgN found that the inhibition of CXCR4 by the drug actually plays a dual role - It increases the neutrophil count in the blood through their release from the lungs, while simultaneously promoting their retention in the blood stream. Discovery of this additional mode of action not only provides a deeper understanding on the drug's mechanism, it also contributes to a more effective utilization of the drug. The ground-breaking study creates the possibility of using a combined drug treatment to maximise release of white blood cells from both the bone marrow and the lungs. The approach may be more effective in reducing the risk of bacterial infections in neutropenic patients.
The team leader, Dr Ng Lai Guan from SIgN said, "We have identified the precise mechanisms of plerixafor treatment, which has important implications on its usage. We can understand through this study the effectiveness or limitations of the drug on certain patients and thereafter craft new clinical approaches to better treat them. Our study forms a conceptual framework to establish improved therapeutic strategies for neutropenia."
Acting Executive Director of SIgN, Associate Professor Laurent Renia, said, "Basic research as such is important for us to fully understand how drugs work, so that we can put them to best use. This is a study which can potentially be translated into clinical applications to impact the health and lives of neutropenic patients."
(1) The fatal whole-body infection is also known as Sepsis.
(2) Neutrophils are the most common type of white blood cells, comprising of 50 - 70 % of the white blood cells. They form an essential part of the immune system and are amongst the first immune cells to arrive at sites of infection.
Notes for editor:
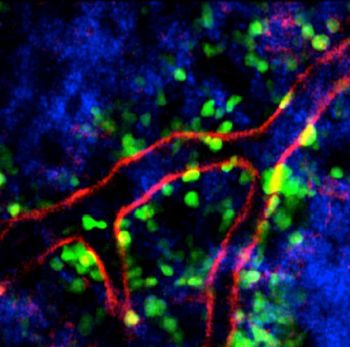
The research findings described in this media release can be found in the September 30, 2013 issue of the Journal of Experimental Medicine (JEM), under the title, "Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from Snapshot of the skull's bone marrow microenvironment, showing neutrophils (green) entering the blood stream through the blood vessel wall (red). (Image by Ng Lai Guan, SIgN) lung demargination and blockade of neutrophil homing to the bone marrow" by Sapna Devi1*, Yiline Wang,1*, Weng Keong Chew,1*, Ronald Lima,2, Noelia A-Gonzalez,3, Citra N.Z. Mattar,4, Shu Zhen Chong,1, Andreas Schlitzer,1, Nadja Bakocevic,1, Samantha Chew,1, Jo L. Keeble,1, Chi Ching Goh,1, Jackson L.Y. Li,1, Maximilien Evrard,1, Benoit Malleret,1, Anis Larbi,1, Laurent Renia,1, Muzlifah haniffa1,5, Suet Mien Tan,6, Jerry K.Y. Chan,4,7,8, Karl Balabanian,9, Takashi Nagasawa,10,11, Francoise Bachelerie,9, Andrrs Hidalgo,3, Florent Ginhoux,1, Paul Kubes,2, and Lai Guan Ng,1.
1. Singapore Immunology Network (SIgN), A*STAR (Agency for Science, Technology and Research), Biopolis, 138648 Singapore
2. Calvin, Phoebe, and Joan Snyder Institute for Infection, Immunity, and Inflammation, University of Calgary, Alberta T2N 1N4, Canada
3. Department of Epidemiology, Atherothrombosis and Imaging, Fundacion Centro Nacional de Investigaciones Cardiovasculares (CNIC), 28029 Madrid, Spain
4. Experimental Fetal Medicine Group, Yoo Loo Lin School of Medicine, National University of Singapore, 119228 Singapore
5. Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne NE2 4HH, England, UK
6. School of Biological Sciences, Nanyang Technological University, 637551 Singapore
7. Department of Reproductive Medicine, KK Women's and Children's Hospital, 229899 Singapore
8. Cancer and Stem Cell Biology Program, Duke-NUS Graduate Medical School, 169857 Singapore
9. Inserm Unite Mixte de Recherche (UMR) S996, Universitei Paris-Sud, Laboratory of Excellence in Research on Medication and Innovative Therapeutics, 92140 Clamart, France
10. Department of Immunobiology and Hermatology, Institute for Frontier Medical Sciences, Kyoto University, Kyoto 606-8507, Singapore
11. Japan Science and Technology Agency (JST), Core Research for Evolutional Science and Technology (CREST), Tokyo 102-0076, Japan
*These authors contributed equally to this work
Full text of the article can be accessed from http://www.jem.org/cgi/doi/10.1084/jem.20130056,
About the Singapore Immunology Network (SIgN)
The Singapore Immunology Network (SIgN), officially inaugurated on 15 January 2008, is a research consortium under the Agency for Science, Technology and Research (A*STAR)'s Biomedical Research Council. The mandate of SIgN is to advance human immunology research and participate in international efforts to combat major health problems. Since its launch, SIgN has grown rapidly and currently includes 250 scientists from 26 different countries around the world working under 28 renowned principal investigators. At SIgN, researchers investigate immunity during infection and various inflammatory conditions including cancer and are supported by cutting edge technological research platforms and core services.
Through this, SIgN aims to build a strong platform in basic human immunology research for better translation of research findings into clinical applications. SIgN also sets out to establish productive links with local and international institutions, and encourage the exchange of ideas and expertise between academic, industrial and clinical partners and thus contribute to a vibrant research environment in Singapore. For more information about SIgN, please visit www.sign.a-star.edu.sg.
Contact:
Tan Yun Yun (Ms)
Senior Officer, Corporate Communications
Agency for Science, Technology and Research
Tel: +65 6826 6273
Email: tan_yun_yun@a-star.edu.sg
Topic: Research and development
Source: A*STAR
Sectors: Science & Research
https://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2024 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|