|
|
|
|
|
|
| A new type of sensor device from the German health technology company Digital Diagnostics AG can be used for immediate tests for the new SARS coronavirus. In comparison to currently available rapid tests, the SARS-CoV-2 MEMS 5 Minute Test(TM) directly detects the virus over the entire course of the infection and can be read immediately, without the need for a laboratory. |
Mainz, Germany, Apr 8, 2020 - (ACN Newswire) - A new type of sensor device from the German health technology company Digital Diagnostics AG can be used for immediate tests for the new SARS coronavirus. The SARS-CoV-2 MEMS 5 Minute Test(TM) is created as a pocket-size lab and can be used in four steps anywhere on-site by general practitioners, paramedics and nursing staff without any training. The first prototypes of the biosensor will be available by end of April with first production devices by mid-May.
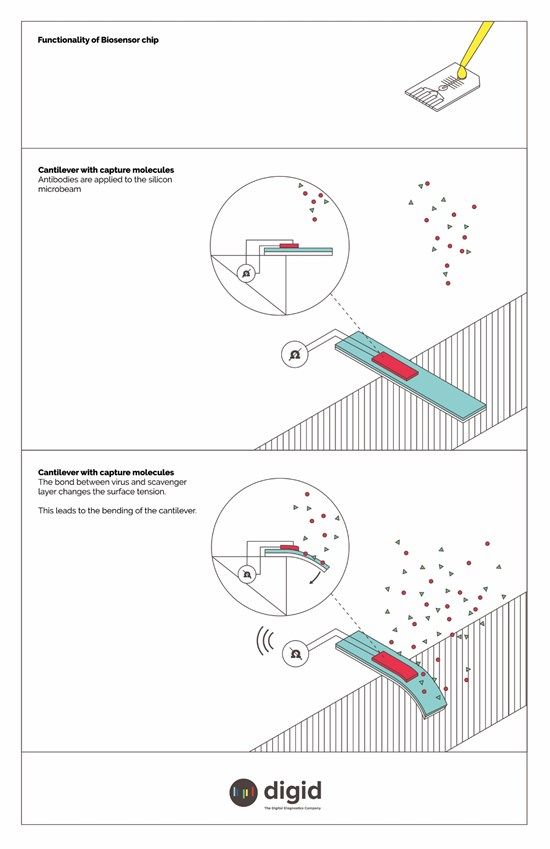 | | Functionality of the biosensor chip (illustrative) - The SARS-CoV-2 MEMS 5 Minute Test(TM) is based on cantilevers, which are integrated onto a microchip with the size of a fingernail. These nanomechanical spring bars made of silicon are extremely thin and can be bent by the application of even a tiny force. This makes them highly sensitive biochemical sensors. (Copyright: Digital Diagnostics AG) |
Compared to previously known rapid tests looking for the presence of antibodies in the test-liquid, the SARS-CoV-2 MEMS 5 Minute Test(TM) leads to a clear "YES" or "NO" result regarding the presence of the actual virus in the test fluid and eliminates the time and cost consuming transport of samples to a laboratory. The great advantage of this method is that the virus can be detected directly with a very high accuracy. In contrast, all other rapid tests known to date are based on the detection of antibodies. But antibodies are not detectable in plasma until 7 to 10 days after infection.
With the 5 Minute Test developed by digid, the SARS-CoV-2 virus can be measured using micro-electro-mechanical systems (MEMS) without the need for time-consuming sample preparation. The SARS-CoV-2 MEMS 5 Minute Test(TM) is based on cantilevers, which are integrated onto a microchip with the size of a fingernail. These nanomechanical spring bars made of silicon are extremely thin and can be bent by the application of even a tiny force. This makes them highly sensitive biochemical sensors. The cantilevers are coated with a capture layer of antibodies. When applied to the chip, these antibodies bind any viruses contained in the test fluid. When the SARS-CoV-2 virus binds to the capture layer, changes in surface tension cause a mechanical bending of the cantilevers, which generates an electrical signal on the chip.
By connecting the digital sensor to a secure analytics platform, the numerous sensor data can be linked with geodata and processed anonymously for big-data applications. This would enable the creation of truly accurate tools to detect emerging regional hotspots of virus spread in near-real-time. Newly emerging chains of infection can then be effectively contained. Restrictions on freedom of movement could also be much more flexible and regionally limited, and the scarce resources of health administrations and hospitals could be used much more purposefully and efficiently.
Press contact
Thomas Huber
semanticom GmbH
+49 151 14 96 58 10
digid-pr@semanticom.eu
Related Files
- digid SARS-CoV-2 MEMS Rapid Test - EN-crv.jpg https://www.newsfilecorp.com/redirect/xpO2UMPe
Related Links
- Read our story on medium.com https://www.newsfilecorp.com/redirect/7kePhJE8
- LinkedIn https://www.newsfilecorp.com/redirect/er4AIJ0o
To view the source version of this press release, please visit https://www.newsfilecorp.com/release/54256
Topic: Press release summary
Source: Digital Diagnostics AG
Sectors: BioTech, Healthcare & Pharm
https://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2025 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|
|
|