KAWASAKI, Japan, Aug 26, 2024 - (JCN Newswire) - Fujitsu today announced that it will begin initiatives to attract global clinical trials to Japan and tackle the ‘drug loss’ issue by working with pharmaceutical companies and medical institutions to build a new medical data ecosystem. The efforts come under the Fujitsu Uvance Healthy Living, which aims to improve people’s well-being.
In July 2024, Fujitsu formed a strategic partnership with Paradigm Health, Inc., a US startup that provides one of the world’s most advanced clinical trial platforms. Through utilizing this platform, Fujitsu’s Healthy Living Platform, and the Fujitsu Kozuchi AI service, Fujitsu and Paradigm will facilitate the collection and processing of data stored at medical institutions and speed up the clinical trial planning process.
In addition, starting today, Fujitsu will release Patient-centric Clinical Trials, a new service for automatically creating clinical trial documents using Fujitsu’s proprietary large language model (LLM). This service will be positioned in the company’s Healthy Living portfolio.
Fujitsu will continue to expand its support to include not only clinical trial planning, but also implementation, and will work to solve issues throughout the entire clinical trial process. Through these efforts, Fujitsu aims to secure 20.0 billion yen in revenue in fiscal 2030, as well as contribute to achieving a society in which patients can quickly obtain the medicine they need and choose the treatment that best suits them.
Background
In Japan, patients who participate in clinical trials are dispersed across multiple hospitals making the process costly and time consuming. There has been an increasing number of cases in which Japan is excluded from regions targeted for global clinical trials. The impact of Japan’s drug pricing policy is also contributing to this situation.
As a result, the number of drugs that are in use outside of Japan but are not approved for use in Japan had risen to 143 as of March 2023 (source: the Japanese Ministry of Health, Labour and Welfare of Japan).
Overview of the Initiatives
1. Accelerating Digitization in the Area of Clinical Trials through Fujitsu’s Partnership with Paradigm
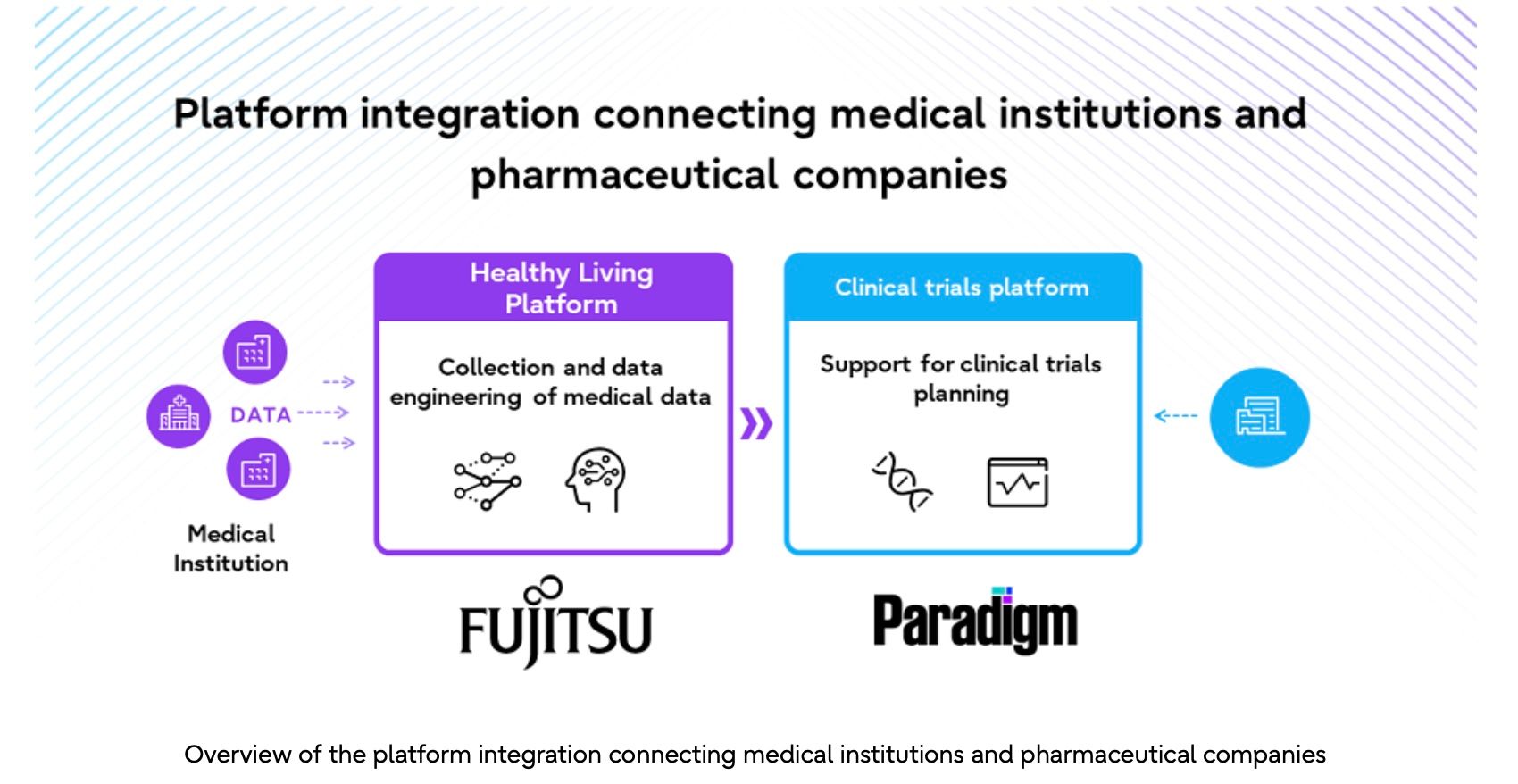
Fujitsu will work with Paradigm, a US startup that provides pharmaceutical companies and medical institutions with one-stop support for the clinical trial process, to develop a new clinical trial data ecosystem that utilizes medical data. Paradigm’s clinical trial platform, which hosts many global clinical trials and has a long track record of success, will be combined with Fujitsu’s operational support expertise and cutting-edge technologies in the area of healthcare and life sciences in Japan.
Clinical data, such as medical data and genomic data, will be collected from medical institutions through Fujitsu’s Healthy Living Platform. Fujitsu’s AI service, Fujitsu Kozuchi, will process this data so that it is compliant with the necessary laws and regulations, and it will be provided to Paradigm. In addition, Paradigm will use its clinical trial platform to analyze the data and provide pharmaceutical companies with the insight needed to plan and conduct clinical trials. This will allow pharmaceutical companies to better understand the situation at the medical institutions as well as the distribution of patients during the planning phase of the clinical trial, and significantly optimize the clinical trial design process.
Medical institutions will also be able to quickly access information about clinical trials in which patients are able to participate through Paradigm’s clinical trial platform, making it easier for medical institutions to encourage patients to participate in clinical trials at the appropriate time.
Fujitsu will launch these solutions starting in September 2024, beginning with providing support to core clinical research hospitals.
2. Providing Patient-centric Clinical Trials, a New Offering for Optimizing Clinical Trial Workflows through a Specialized LLM
In Japan, the hundreds of clinical trial-related documents that pharmaceutical companies need to produce to develop a new drug are still being created and managed manually. However, by using its LLM specialized for clinical trials, Fujitsu will offer a service that can automatically create these documents and ensure that they are in compliance with regulations. This service will be provided in Japan as the Patient-centric Clinical Trials offering as part of its Healthy Living portfolio.
In conducting a PoC of this offering with a pharmaceutical company, the LLM was able to automatically create approximately 80% of each document. According to Fujitsu’s estimates, this is expected to lead to a reduction in the total time required to create documentation of up to 50%. Through this offering, Fujitsu aims to make a significant contribution to optimizing the clinical trial planning and implementation workflow.
Future Plans
Fujitsu will work to accelerate the digitalization of the clinical trial environment in Japan by providing total support for the entire clinical trial process, including planning and implementation. Through these efforts, Fujitsu aims to create the groundwork for increasing the number of global clinical trials conducted in Japan, solve the issue of drug loss and contribute to achieving a society in which all individuals can choose to live their lives in the way that suits them.
Statement from Kent Thoelke, CEO of Paradigm Health, Inc.
Paradigm is excited to be partnering with Fujitsu to maximize clinical trial efficiency in Japan. This collaboration will modernize the clinical trial model and provide unprecedented access to clinical trials for patients across Japan. The partnership will leverage Paradigm’s industry leading Platform and Fujitsu’s expansive healthcare solutions to increase clinical trial patient accrual, decrease overall drug development timelines and costs, while working to eliminate drug loss in Japan. We believe that this collaboration will establish Japan as an essential part of the global clinical trials and drug development ecosystem while ensuring that Japanese patients have equitable access to the best possible care options.
About Fujitsu
Fujitsu’s purpose is to make the world more sustainable by building trust in society through innovation. As the digital transformation partner of choice for customers in over 100 countries, our 124,000 employees work to resolve some of the greatest challenges facing humanity. Our range of services and solutions draw on five key technologies: Computing, Networks, AI, Data & Security, and Converging Technologies, which we bring together to deliver sustainability transformation. Fujitsu Limited (TSE:6702) reported consolidated revenues of 3.7 trillion yen (US$26 billion) for the fiscal year ended March 31, 2024 and remains the top digital services company in Japan by market share. Find out more: www.fujitsu.com.
Press Contacts
Fujitsu Limited
Public and Investor Relations Division
Inquiries
Topic: Press release summary
Source: Fujitsu Ltd
Sectors: Enterprise IT, Artificial Intel [AI], MedTech
https://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2026 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|