HONG KONG, Feb 11, 2025 - (ACN Newswire) - On Feb. 9, 2025, the official website of the Center for Drug Evaluation, National Medical Products Administration of China indicates that the Biologics License Application (BLA) for Luzhu Biotech’s (2480.HK) biological product for prophylaxis, Recombinant Herpes Zoster Vaccine (CHO Cells), has been accepted. This vaccine is the second recombinant herpes zoster vaccine worldwide—following GSK’s Shingrix—and the first of its kind to be submitted for market approval in China.
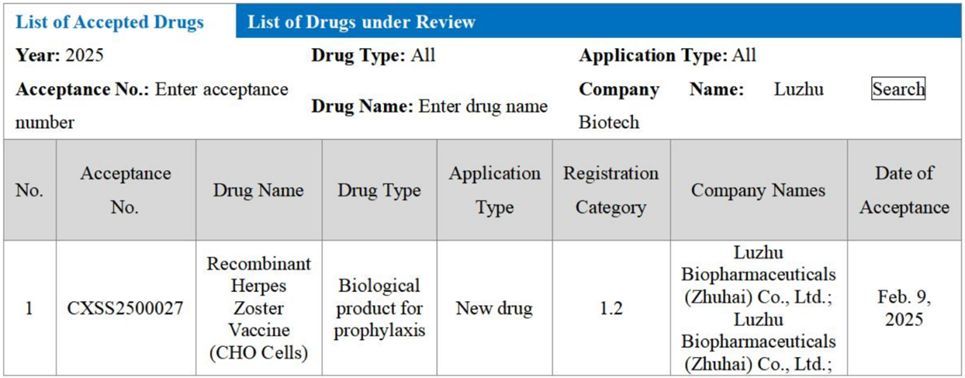
Translated version
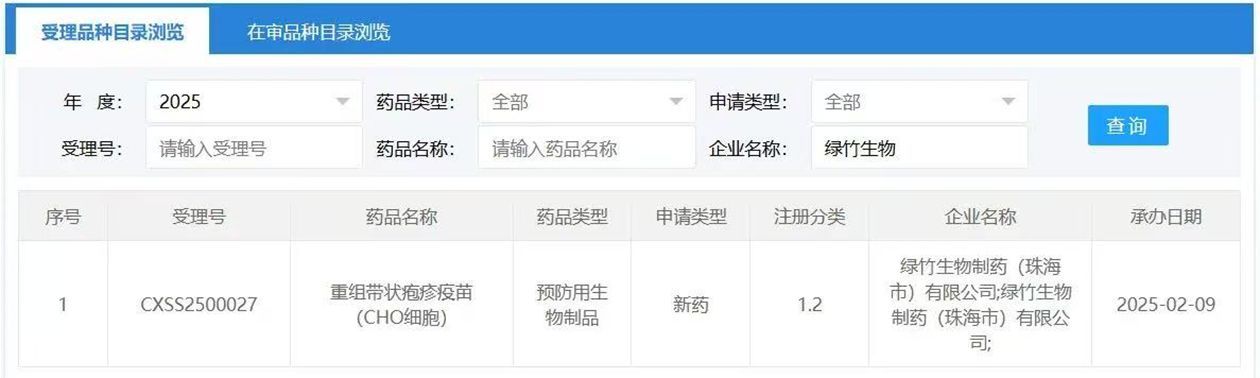
Origin version
01
How Big Is the Herpes Zoster Vaccine Market?
BCHT’s Ganwei is currently the only domestically marketed herpes zoster vaccine in China. Since its launch in 2023, it has generated nearly RMB900 million in sales within the year, drawing significant market attention and rapidly intensifying competition in the field. As of Dec. 31, 2024, over 20 companies in China have advanced their herpes zoster vaccine pipelines into clinical trials or received Investigational New Drug (IND) approvals—an increase from just 4 companies in 2022.
In 2023, Zhifei Biological began distributing vaccine Shingrix in China imported from GSK. Shingrix was approved for the Chinese market in less than 3 months through the “Green Channel” in 2019 and was commercialized the following year. As a blockbuster vaccine, Shingrix achieved approximately USD4.2 billion in global sales in 2024, ranking third worldwide.
Ganwei and Shingrix represent two distinct technological approaches to herpes zoster vaccines, each with clear advantages and disadvantages. However, do their respective shortcomings in protection efficacy and side effects limit their broader market adoption? Could a new vaccine that combines the strengths of both while addressing their weakness further expand the herpes zoster vaccine market? If approved, Luzhu Biotech’s innovative Recombinant Herpes Zoster Vaccine could potentially be the breakthrough product that achieves this goal.
02
Luzhu Biotech’s Path to Innovation in Herpes Zoster Vaccines
Founded in 2001, Luzhu Biotech has been one of China’s leading innovators in vaccine development since its establishment. Before 2008, the company successfully developed three types of bacterial polysaccharide conjugate vaccines and two multivalent meningococcal polysaccharide vaccines, including but not limited to the world’s first AC-Hib conjugate vaccine, the liquid AC polysaccharide conjugate vaccine, and China’s first group ACYW135 meningococcal polysaccharide vaccine.
As Luzhu Biotech’s most advanced vaccine project, the company initiated the development of its recombinant herpes zoster vaccine in 2018. After 7 years of research, it has now reached the regulatory submission stage. Globally, only four herpes zoster vaccines are currently on the market—aside from Shingrix, the other three are live-attenuated vaccines.
Following the same recombinant protein technology pathway, Luzhu Biotech’s herpes zoster vaccine has undergone rigorous head-to-head comparisons with Shingrix in preclinical studies and Phases 1 and 3 clinical trials. According to publicly disclosed data, Luzhu Biotech’s vaccine has demonstrated superior cellular immune response and a more favorable safety profile compared to Shingrix.
Can this vaccine help Luzhu Biotech, a Hong Hong-listed Chapter 18A company, turn profitable and bring substantial revenue? Could it even become a globally recognized blockbuster? Notably, Luzhu Biotech’s herpes zoster vaccine is also undergoing clinical trials in the United States, making it the first Chinese vaccine to be submitted for approval in both China and the United States. If it surpasses Shingrix in overall effectiveness, including protection and safety, it could emerge as the best-in-class product, naturally paving the way for its success in the global market.
Whether Luzhu Biotech can inject new vitality into the global vaccine market remains an exciting prospect to watch.
PEANUT MEDIA LIMITED
Ms. Chen
Direct Line: +86-755-61619798 x8210
http://peanutmedia.com/
Topic: Press release summary
Source: PeanutMedia
Sectors: eSports, Gaming
https://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2026 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|